007 Water: The Magic of Life
When it comes to watering plants, there’s really only three options - too much, too little, or just right … but there’s the rub. Just like with light and temperature requirements, all plants are different. Some plants, like succulents, can get by with pretty dry soil and some aquatic plants literally live in water. There’s a whole spectrum of water needs in between.
Key Concepts
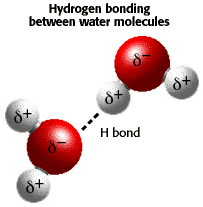
Water is a "polar" molecule, meaning that there is an uneven distribution of electron density. Water has a partial negative charge near the oxygen atom due the unshared pairs of electrons, and partial positive charges near the hydrogen atoms.
An electrostatic attraction between the partial positive charge near the hydrogen atoms and the partial negative charge near the oxygen results in the formation of a hydrogen bond as shown in the illustration.
Hydrogen bonding between water molecules
The ability of ions and other molecules to dissolve in water is due to polarity. For example, in the illustration below sodium chloride (NaCl) is shown in its crystalline form and dissolved in water.
This ability for water to dissolve polar molecules like salts is essential for transporting sugars and nutrients throughout plants. Transpiration is the process of water movement through a plant and its evaporation from aerial parts, such as leaves, stems and flowers. Water is necessary for plants but only a small amount of water taken up by the roots is used for growth and metabolism.
Greenhouse Watering Systems
Garden Wisdom
If there is magic on this planet, it is contained in water.
-Loren Eiseley